|
The discovery of
voltage-gated ion channels (VGICs) in the dendrites of
various neurons constitutes an important breakthrough in
neuroscience research. These VGICs express with a wide
range of subcellular localization profiles across the
somatodendritic arbor, and undergo localized or global
plasticity under several physiological and
pathophysiological conditions. Recent findings suggest
that changes in VGICs and the consequent changes in
intrinsic properties, apart from and in conjunction with
traditionally considered synaptic changes, could play
important roles in encoding information in a single
neuron. Against this, research in our laboratory is
focused on understanding the physiological roles of the
various VGICs that are expressed in neuronal dendrites.
The overall goal is to understand the roles of the
presence and plasticity of VGICs in terms of
spatiotemporal interactions with each other, and how they
enable neural coding and signal propagation in single
neurons. We employ a combination of experimental (in
vitro and in vivo electrophysiology and
imaging from the rat hippocampus) and computational
techniques to address questions that arise towards
achieving this goal.
Emergence
of functional maps within a single neuron
Several VGICs express
subcellular gradients in their expression profiles and
mediate gradients in physiological measurements that have
been referred to as “functional maps” within a neuron (Narayanan
and Johnston, J. Neurophysiology, 2012).
Assessment of spatial interactions among ion channels is
critical from the standpoint of how such functional maps
emerge from gradients in VGIC conductances, especially
given the complex dendritic morphology associated with
neurons.
To assess spatial
interactions among ion channels, we developed a
generalized quantitative framework (that we referred to as
“influence field”) to analyze the extent of influence of a
spatially localized VGIC conductance on different
physiological properties along the stretch of a neuron (Rathour
and Narayanan, J. Neurophysiology, 2012).
Employing this framework, we reconstructed functional maps
of specific physiological measurements from VGIC
conductance gradients. Analyses of these reconstructions
revealed that the cumulative contribution of VGIC
conductances in adjacent compartments plays a critical
role in the emergence of functional maps within a single
neuron. Additionally, we also demonstrated that these
functional maps cease to exist if the dendritic arbor were
significantly atrophied (Dhupia et al., Frontiers
in Cellular Neuroscience, 2015). These
results have important implications for neuronal
physiology under pathological conditions where significant
dendritic atrophy has been reported.
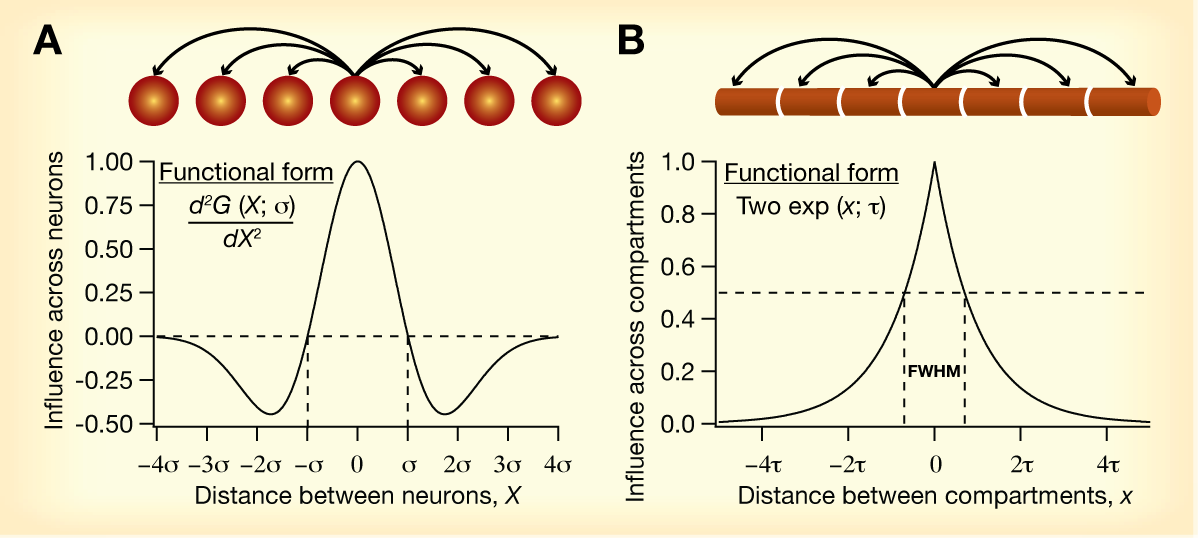
A direct analogy for how
influence fields could be employed in models of single
neuron function (e.g., neural coding, learning)
could be drawn from models of cortical circuitry employed
in the sensory map literature. In sensory map models, a
Mexican-hat function (panel A above) is used as an
abstraction for intracortical connectivity between neurons
in the model network. Such interactions have been
demonstrated to be extremely critical in the
self-organized formation of sensory maps, and are
responsible for the conversion of local computations by
simplified neuronal units to global order. Influence
fields could be envisioned as a mechanism for
intercompartmental interactions within a neuron (Panel B
above), as opposed to Mexican-hat interactions across
neurons. Here, given the existence of local plasticity in
VGICs, it becomes important to assess the roles that such
local computations play and analyze if these could
translate into global order within the neuron — such as
gradients in specific ion channel densities across the
neuronal arbor.
Related references
Neha Dhupia, Rahul Kumar
Rathour and Rishikesh Narayanan,
Dendritic atrophy constricts functional maps in resonance
and impedance properties of hippocampal model neurons, Frontiers
in Cellular Neuroscience, 8, 456: 1-17, January
2015. [PDF file]
[Article
at publisher's site]
Rahul Kumar Rathour and
Rishikesh Narayanan, Influence
fields: A quantitative framework for the representation
and analysis of active dendrites, Journal of
Neurophysiology, 107(9), 2313–2334, May 2012. [PDF
File] [Link
to paper at publisher's site]
Rishikesh
Narayanan and Daniel Johnston, Functional maps
within a single neuron, Journal of Neurophysiology,
108(9), 2343–2351, November 2012. [PDF
file] [Link
to paper at publisher's site]
Variability,
interactions and intrinsic response dynamics
Hippocampal neurons reside within an oscillating neuronal
network. These oscillations span multiple frequency
ranges, sometimes with each frequency range reflective of
a specific behavioral state of the animal. Intrinsic
response dynamics (IRD) constitute the manner in which a
single neuron intrinsically responds to such oscillatory
inputs emerging from differential spatiotemporal patterns
of activation. Whereas the spatial aspect is governed by
the dendritic locations of the input stimuli, the temporal
aspect is dictated by the arrival times of synaptic
inputs. In addition to providing a detailed picture of
neuronal information processing, IRD also offers an
alternate, and much less-explored, cellular correlate for
learning and memory. The passive properties of the
dendritic tree in conjunction with the densities and
characteristics of different VGICs located at various
dendritic locations mediate the IRD of a neuron. Given the
ability of VGICs to amplify or suppress specific input
frequencies, it has been emerging from recent results that
these channels can sculpt IRD in a manner suitable for the
neuron and its network, through variable expression and/or
activity-dependent plasticity.
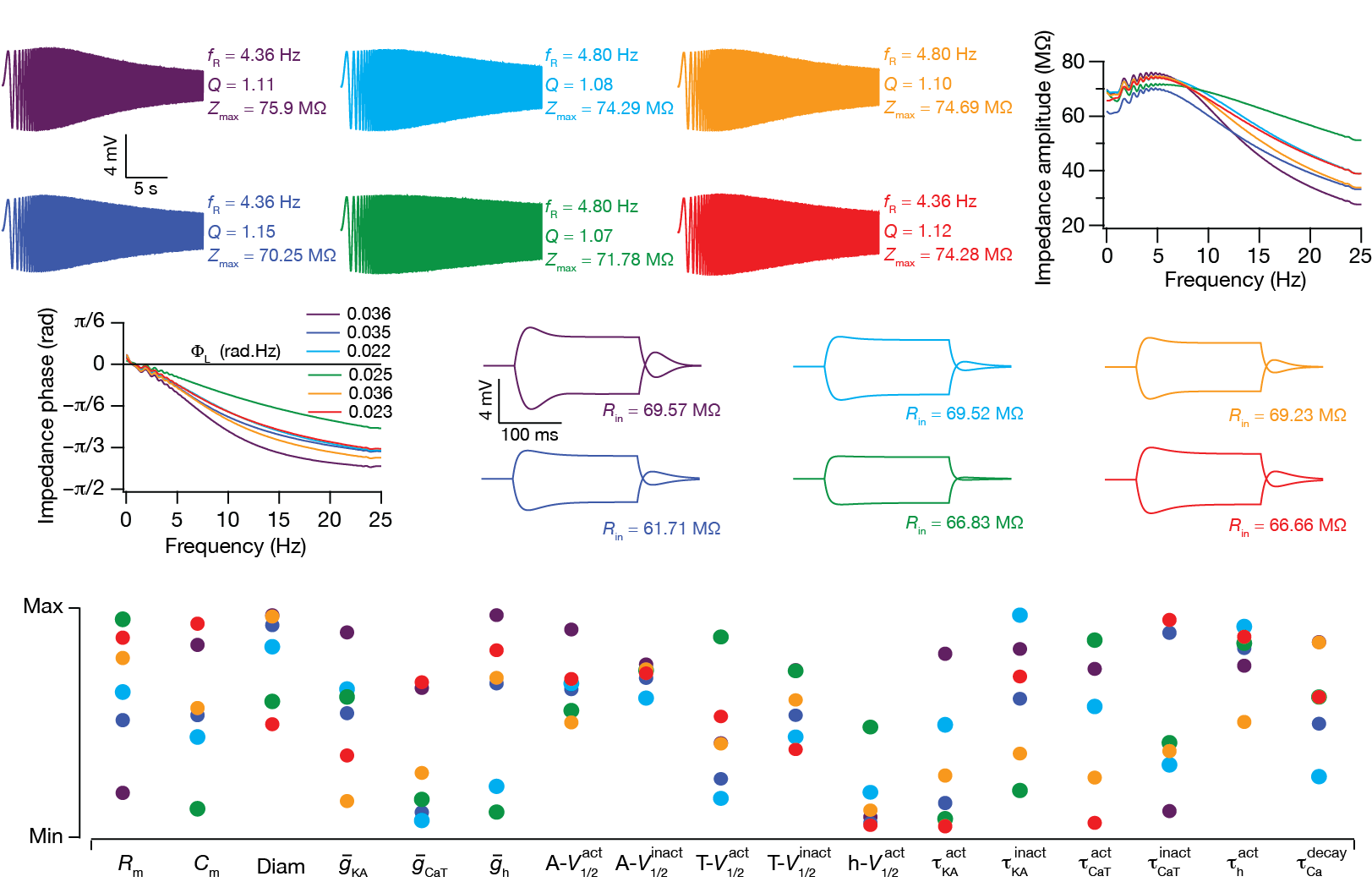
We have systematically analyzed the roles
of the two inactivating subthreshold VGICs (A-type
K+ and T-type Ca2+),
individually and in various combinations with the
noninactivating h conductance, in regulating
several physiological IRD measurements. We found that the
coexpression of the h and T-type Ca2+
conductances augmented the range of parameters over which
they sustained resonance and inductive phase lead.
Additionally, coexpression of the A-type K+
conductance with the h or the T-type
Ca2+ conductance elicited changes in IRD
measurements that were similar to those obtained with the
expression of a leak conductance with a resonating
conductance. Further, we employed the global sensitivity
of IRD measurements to all parameters associated with
models expressing all three VGICs, and found that
functionally similar models could be achieved even when
underlying parameters displayed tremendous variability and
exhibited weak pair-wise correlations. Shown in the above
figure are six color-coded model neurons displaying very
similar IRD measurements (panels A–D
above) despite tremendous variability in underlying
parameters (panel E above). Based on these
results, we postulate that the differential expression and
activity-dependent plasticity of these VGICs contribute to
robustness of subthreshold IRD, whereby response
homeostasis is achieved by recruiting several non-unique
combinations of these channel parameters (Rathour and
Narayanan, J. Physiology, 2012).
How do neurons with complex morphologies maintain these
functional maps despite constant turnover of and
plasticity in the several ion channels that mediate them?
We addressed this question within a global sensitivity
analysis framework spanning channels and measurements from
the cell body and dendrites of hippocampal neurons. Our
results demonstrated that individual channel properties or
their densities need not be maintained at constant levels
in achieving overall homeostasis of several coexistent
functional maps. We suggested collective channelostasis,
where several channels regulate their properties and
expression profiles in an uncorrelated manner, as an
alternative for accomplishing homeostasis of functional
maps (Rathour and Narayanan, PNAS (USA), 2014).
Although these studies explored the role of interactions
among ion channels in regulating IRD under in vitro
conditions, the impact of sustained high-conductance
states (observed under in vivo conditions) on
these interactions has not been assessed. To fill this
lacuna, we assessed the impact of interactions between HCN
and A-type K+ channels on several
measures of intrinsic excitability. We found that
high-conductance states and A-type K+
channels are potential regulators of the
conductance-current balance triggered by the presence of
HCN channels (Mishra and Narayanan, J.
Neurophysiology, 2015). These results
together suggested that intrinsic response dynamics of
neurons under physiological and pathophysiological
neuronal states are critically reliant on interactions
among several subthreshold channels and on
high-conductance states.
Related
references
Poonam Mishra and Rishikesh
Narayanan, High-conductance states and A-type
K+ channels are potential regulators of the
conductance-current balance triggered by HCN channels, Journal
of Neurophysiology, 113(1): 23-43, January 2015. [PDF
File] [Article
at publisher's site]
Rahul Kumar Rathour and Rishikesh
Narayanan, Homeostasis of functional maps in
active dendrites emerges in the absence of individual
channelostasis, Proceedings of the National Academy
of Sciences (USA), 111(17), E1787-E1796, April
2014. [PDF File] [Link
to paper at publisher's site]
Rahul Kumar Rathour and Rishikesh
Narayanan, Inactivating ion channels augment
robustness of subthreshold intrinsic response dynamics to
parametric variability in hippocampal model neurons, The
Journal of Physiology (London), 590 (22),
5629–5652, November 2012. [PDF
File] [Supplementary
PDF File] [Link
to paper at publisher's site]
Rahul Kumar Rathour, Ruchi Malik and Rishikesh
Narayanan, Transient potassium channels augment
degeneracy in hippocampal active dendritic spectral
tuning, Scientific Reports, 6, 24678: 1-14,
April 2016. [PDF File]
[Supplementary PDF File]
[Article
at publisher's site]
Active
dendrites, spectral selectivity & coincidence
detection
How does the presence of plastic active dendrites in a
pyramidal neuron alter its spike initiation dynamics? To
answer this question, we measured the spike-triggered
average (STA) from experimentally constrained,
conductance-based hippocampal neuronal models of various
morphological complexities. We transformed the STA
computed from these models to the spectral and the
spectrotemporal domains and found that the spike
initiation dynamics exhibited temporally localized
selectivity to a characteristic frequency. In the presence
of the hyperpolarization-activated cyclic nucleotide-gated
(HCN) channels, the STA characteristic frequency strongly
correlated with the subthreshold resonance frequency in
the theta frequency range. Increases in HCN channel
density or in input variance increased the STA
characteristic frequency and its selectivity strength. In
the absence of HCN channels, the STA exhibited weak delta
frequency selectivity and the characteristic frequency was
related to the repolarization dynamics of the action
potentials and the recovery kinetics of sodium channels
from inactivation.
Comparison of STA obtained with inputs at various
dendritic locations revealed that nonspiking and spiking
dendrites increased and reduced the spectrotemporal
integration window of the STA with increasing distance
from the soma as direct consequences of passive filtering
and dendritic spike initiation, respectively. Finally, the
presence of HCN channels set the STA characteristic
frequency in the theta range across the somatodendritic
arbor and specific STA measurements were strongly related
to equivalent transfer-impedance-related measurements. Our
results identify explicit roles for plastic active
dendrites in neural coding and strongly recommend a
dynamically reconfigurable multi-STA model (below) to
characterize location-dependent input feature selectivity
in pyramidal neurons (Das and Narayanan, J.
Neuroscience, 2015).
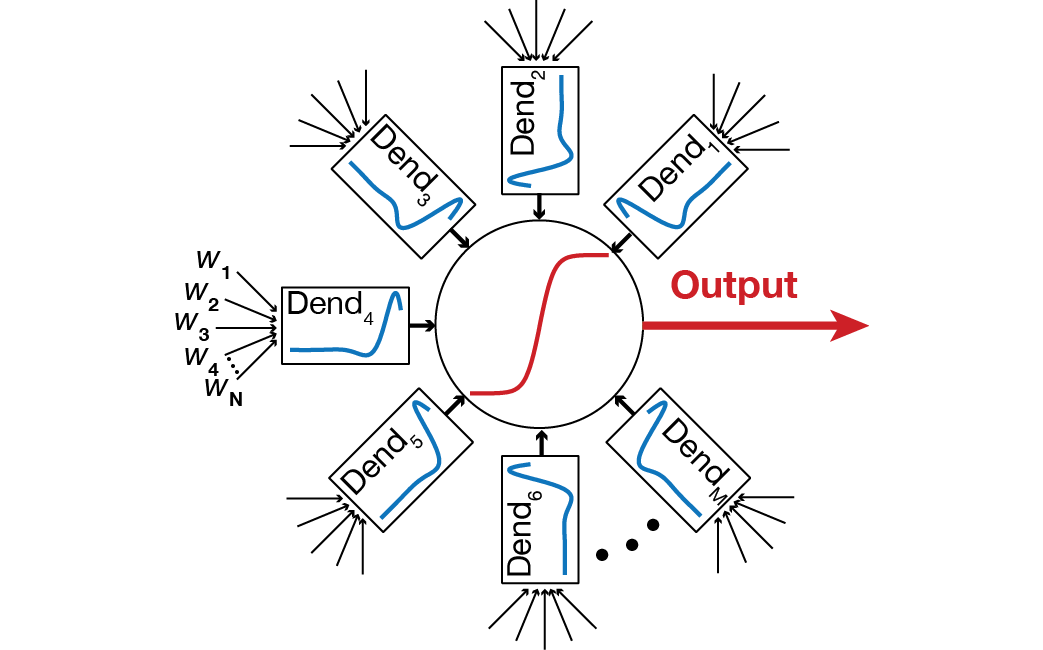
Further, different classes of neurons are
known to depict different forms of STA, with Class I
excitability neurons showing integrator-like properties,
and Class II/III neurons endowed with coincidence
detector-like features. Our results demonstrate that
different parts of a neuron can span the
Integrator-Coincidence Detector continuum depending on the
density of HCN channels. These results together suggest
that neural coding and learning in neurons with plastic
active dendrites should not be viewed from the limited
perspective of synaptic properties and their plasticity,
but should incorporate the profiles and plasticity of
different voltage-gated ion channels and other intrinsic
mechanisms as well. We also extended this analysis to
interactions among several subthreshold VGICs, and found
that spatiotemporal interactions among these channels
critically regulate the STA and coincidence detection
window (CDW) in hippocampal neurons. Specifically, we
showed that the presence of resonating and
spike-generating conductances serve as a mechanism
underlying the emergence of stratified gamma-range
coincidence detection in the dendrites of CA1 pyramidal
neurons (slow-gamma CDW in proximal dendrites and a
fast-gamma CDW in distal dendrites), enabling them to
perform behaviour- and state-dependent gamma frequency
multiplexing (Das and Narayanan, J. Physiology
(London), 2015).
Related references
Anindita Das and Rishikesh
Narayanan, Theta-frequency selectivity in the
somatic spike triggered average of rat hippocampal
pyramidal neurons is dependent on HCN channels, Journal
of Neurophysiology, In Press, August 2017. [PDF
File] [Article
at publisher's site]
Anindita Das and Rishikesh
Narayanan, Active dendrites mediate stratified
gamma-range coincidence detection in hippocampal model
neurons, The Journal of Physiology (London),
593(16): 3549–3576, August 2015. [PDF
File] [Article
at publisher's site]
Anindita Das and Rishikesh
Narayanan, Active dendrites regulate spectral
selectivity in location-dependent spike initiation
dynamics of hippocampal model neurons, The Journal of
Neuroscience, 34(4): 1195-1211, January 2014. [PDF
file]
[Link
to paper at publisher's site]
Anindita Das, Rahul Kumar Rathour
and Rishikesh Narayanan, Strings on a
violin: Location dependence of frequency tuning in
active dendrites, Frontiers in Cellular
Neuroscience, 11, 72: 1-8, March 2017. [PDF
file] [Article
at publisher's site]
Dendritic ion channels
and local field potentials
What are the implications for the existence of
subthreshold VGICs, their localization profiles and
plasticity on local field potentials (LFPs)? We assessed
the role of HCN channels in altering hippocampal
theta-frequency LFPs and associated spike phase. To do
this, we presented spatiotemporally randomized, balanced
theta-modulated excitatory and inhibitory inputs to
somatically aligned, morphologically realistic pyramidal
neuron models spread across a cylindrical neuropil. We
computed LFPs from seven electrode sites and found that
the insertion of an experimentally constrained
HCN-conductance gradient into these neurons introduced a
location-dependent lead in the LFP phase without
significantly altering its amplitude. Further, neurons
fired action potentials at specific theta-phase of the
LFP, and the insertion of HCN channels introduced large
lags in this spike phase and a striking enhancement in
neuronal spike phase coherence. These results uncover
specific roles for HCN channels and their plasticity in
phase coding schemas and in the formation and dynamic
reconfiguration of neuronal cell assemblies (Sinha and
Narayanan, PNAS (USA), 2015).
Related references
Manisha Sinha and Rishikesh Narayanan,
HCN channels enhance spike phase coherence and regulate
the phase of spikes and LFPs in the theta-frequency range,
Proceedings of the National Academy of Sciences (USA),
112(17): E2207-E2216, April 2015. [PDF
File] [Article
at publisher's site]
The Endoplasmic
Reticulum in Neurons and Astrocytes
Neuronal physiology is defined not just by spatiotemporal
interactions amongst plasma membrane VGICs. The
endoplasmic reticulum (ER) spans the entire neuronal
morphology and is endowed with numerous ion channels on
its membrane. Along an active dendritic membrane, this
arrangement constitutes the presence of two continuous
membranes that can modulate and propagate information by
recruiting channels on either of them. Therefore, we
explored interactions between these two membranes and
their implications for neuronal physiology and information
encoding within neurons.
Roles of dendritic ion channels in modulating release
of calcium from the ER stores: The ER membrane is
endowed with inositol triphosphate receptors (InsP3R)
that are permeable to calcium and can sustain active
propagation of calcium waves within neurons. Focusing
specifically on the interactions between the A-type
K+ channels and InsP3Rs, we have
demonstrated that A-type K+ channels
could regulate Ca2+ release through InsP3Rs,
thereby altering propagation of Ca2+ waves and
induction of synaptic plasticity. Our results suggest that
such interactions between conductances on the dendritic
membrane and Ca2+ channels on the ER membrane
could critically regulate biophysical/biochemical signal
integration and steer the spatiotemporal spread of
signaling microdomains within neurons (Ashhad and
Narayanan, J. Physiology, 2012).
Roles of calcium released from the ER in modulating
plasma membrane ion channels: We explored the
counterpart to these interactions, whereby calcium release
through InsP3 receptors can alter the
properties of channels that reside on the plasma membrane.
In this case, we explored the interactions between InsP3
receptors and HCN (h) channels that reside on the
plasma membrane. We showed that the activation of InsP3
receptors through intracellular InsP3 injection
is sufficient to elicit plasticity in neuronal intrinsic
response dynamics through changes in the h
current. We have also shown that this form of plasticity
is dependent on calcium release through InsP3
receptors and the PKA (protein kinase A) pathway (Ashhad
et al., J. Neurophysiol., 2015).
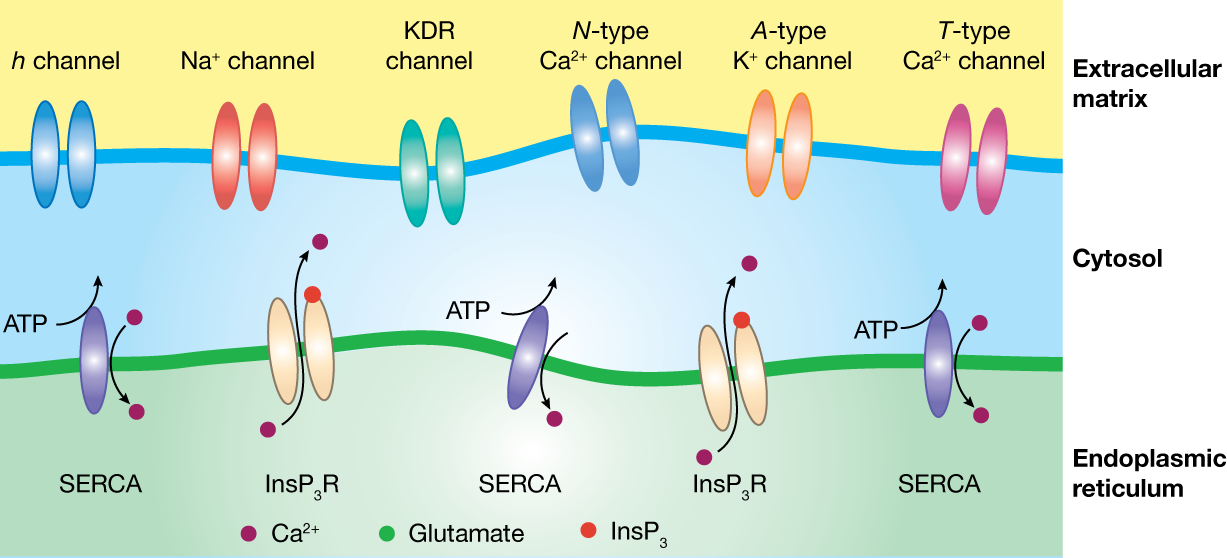
The figure above depicts the two continuous membranes
and typical ion channels present on these membranes. The
ER membrane is endowed with inositol trisphosphate
receptors (InsP3R) that are permeable to
calcium and can sustain active propagation of calcium
waves within neurons. The dendritic membrane depicts
typical ion channels present on the dendrites of
hippocampal CA1 pyramidal neurons, and can sustain
active flow of information.
Glial cells in the brain actively communicate with
neurons through release of transmitter molecules that
result in neuronal voltage deflections, thereby playing
vital roles in neuronal information processing. Although
a significant proportion of information processing in
neurons is performed in their dendritic arborization,
the impact of gliotransmission on neuronal dendrites has
not been mapped. In a study involving dendritic
patch-clamp electrophysiology and paired
neuron-astrocyte recordings, we showed that
gliotransmission, acting through differentially
localized slow receptors, results in strikingly large
voltage deflections in neuronal dendrites, with the
strength and spread of these deflections critically
regulated by dendritic ion channels. Our results add a
significantly complex dimension to neuron–glia
interactions by demonstrating that neuronal dendrites
and their voltage-gated channels play active roles in
regulating the impact of such interactions.
Additionally, these results unveil an important role for
active dendrites in regulating the impact of
gliotransmission on neurons and suggest astrocytes as a
source of dendritic plateau potentials that have been
implicated in localized plasticity and place cell
formation (Ashhad and Narayanan, PNAS (USA),
2016).
Related
references
Sufyan Ashhad, Daniel Johnston and Rishikesh
Narayanan, Activation of InsP3
receptors is sufficient for inducing graded intrinsic
plasticity in rat hippocampal pyramidal neurons, Journal
of Neurophysiology, 113(7): 2002-2013, April 2015.
[PDF File] [Article
at publisher's site]
Sufyan Ashhad and Rishikesh
Narayanan, Quantitative interactions between
the A-type K+ current and inositol
trisphosphate receptors regulate intraneuronal Ca2+
waves and synaptic plasticity, The Journal of
Physiology (London), 591 (7): 1645–1669, April
2013. [PDF file]
[Link
to paper at publisher's site]
Sufyan Ashhad and Rishikesh Narayanan,
Active dendrites regulate the impact of gliotransmission
on rat hippocampal pyramidal neurons, Proceedings of
the National Academy of Sciences (USA), 113(23):
E3280-E3289, June 2016. [PDF
File] [Article
at publisher's site]
Homeostasis through
plasticity interactions
Neurons are dynamic entities with plasticity altering the
density and properties of these VGICs/receptors across the
somatodendritic arbor, with synergistic interactions among
different forms of plasticity. How do neurons maintain
activity homeostasis against such ubiquitous plasticity?
How do they retain specific plasticity profiles despite
tremendous variability in underlying channel properties?
To address the first question, we developed
calcium-dependent plasticity rules for the HCN channels,
and their interactions with calcium-dependent synaptic
plasticity. In doing this, we demonstrated that the
synergy between synaptic and HCN plasticity retains
stability in the synaptic learning system, maintains
firing rate homeostasis and enhances the robustness of
information transfer across the neuron. Our study
established a broad framework for the coexistence of
synaptic and VGIC plasticity in neural systems that are
required to stably encode memory in learning systems (Honnuraiah
and Narayanan, PLoS ONE, 2013).
In answering the second question, we considered
interactions among different channels and receptors
through global sensitivity analysis. Analyzing valid
models that were obtained from this analysis, we found
that similar short- and long-term plasticity profiles
could emerge with several nonunique parametric
combinations and that parameters exhibited weak pairwise
correlations. These results suggested that there are
several nonunique routes to regulate synaptic plasticity
profiles, and plasticity homeostasis could be achieved
through any of these several routes (Anirudhan and
Narayanan, J. Neuroscience, 2015; Mukunda and
Narayanan, J. Physiology, 2017). In
addition, we also showed that neurons can undergo variable
plasticity in several ion channels towards reconciling the
maintenance of calcium homeostasis with perpetual switches
in behavioral-state-dependent afferent synaptic activity (Srikanth
and Narayanan, eNeuro, 2015).
Related
references
Arun Anirudhan and Rishikesh
Narayanan, Analogous synaptic plasticity
profiles emerge from disparate channel combinations,
The Journal of Neuroscience, 35(11): 4691-4705,
March 2015. [PDF File]
[Article
at publisher's site]
Chinmayee L Mukunda and Rishikesh
Narayanan, Degeneracy in the regulation of
short-term plasticity and synaptic filtering by
presynaptic mechanisms, The Journal of Physiology
(London), 595(8): 2611-2637, April 2017. [PDF
File] [Article
at publisher's site]
Sunandha Srikanth and Rishikesh
Narayanan, Variability in state-dependent
plasticity of intrinsic properties during cell-autonomous
self-regulation of calcium homeostasis in hippocampal
model neurons, eNeuro, 2(4), e0053-15.2015:
1-24, August 2015. [PDF
File] [Article
at publisher's site]
Suraj Honnuraiah and Rishikesh
Narayanan, A calcium-dependent plasticity rule
for HCN channels maintains activity homeostasis and stable
synaptic learning, PLoS One, 8(2), e55590:
1–17, February 2013. [PDF
file][Supplementary
PDF File] [Article
at publisher's site]
|



